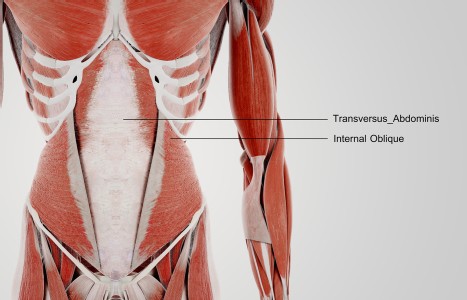
TrA-2, my primary needle location, I needle 95% of the time and I think it works the best. You’ll know you have the right point location when you discover the muscle twitching when applying electric stimulation.
Focus on Research
Research has become a frequently heard buzzword in the acupuncture and Oriental medicine (AOM) community. Practitioners and AOM clinics are asking how to conduct office-based research. Insurance companies, health policy makers, allopathic hospitals, and the National Institutes of Health are requesting to see clinical trials of acupuncture efficacy. Acupuncture colleges are beginning to hire research directors.
Why is this happening? Isn't this a form of medicine that has been patiently and empirically honed in the forge of experience over several millennia? Then why is it now being asked to "prove itself" by designing trials comparing needling at acupoints to needling at non-point sites; comparing herbal formulas to placebo herbs; or comparing the effectiveness of acupuncture to biomedical standard care?
This apparent paradox reflects a major change which began some 50 years ago in the way the allopathic medical profession and governmental regulatory agencies decide which treatments are effective and should be approved for use.
Prior to the 1950s, "experience-based medicine" -- whether grounded in cellular and molecular views in the West or in energetic systems in the East -- was the accepted form of practice. For the most part, what "worked" was retained, refined and recorded in medical texts and materia medica; what didn't work was discarded. After World War II, mainstream health care in the West began a significant shift from empirical practice to "evidence-based medicine." An expectation arose that the value of every drug, device or surgical procedure would be rigorously demonstrated in scientific trials prior to acceptance for use in clinical practice.
The shift from "experience" to "evidence" was in large part a consequence of the rise of pharmaceutical companies in the U.S. and Europe as a means of putting teeth into the medical credo, "First, do no harm". (The appearance of severe birth defects among women prescribed the undertested drug thalidomide to treat morning sickness provided a dramatic impetus in the late 1950s for strengthening the Food and Drug Administration's oversight of drug testing.) The largely unbounded (and ungrounded) faith of that era in drugs as "magic bullets" enhanced a patriarchal, healing-from-without model of medicine that cast a shadow over health care practices like Oriental medicine that accepted and encouraged healing from within.
It was a logical step for health care regulatory agencies and insurance providers to require that treatment modalities other than those within allopathic medicine meet the same rigorous testing requirements prior to clinical use and reimbursement. In the early 1990s, for example, when AOM practitioners began to lobby insurance companies to extend coverage for their services, the companies balked since the FDA was still classifying acupuncture needles as experimental devices. This led to the (successful) petitioning of the FDA to upgrade the status of sterile, single-use needles to class II safe and effective devices.
Once the needles were government approved, it was the task of the National Institutes of Health to review the practice of acupuncture. The approach they chose, the consensus conference, is a well-established format in which new medical treatments and procedures are evaluated by a panel of prominent physicians and scientists. The acupuncture community was taken aback. How could their practice be fairly evaluated within a two-day format? The NIH's response was a reminder of their commitment to "evidence." They would examine only controlled clinical trials and basic research studies, not medical records or testimonials of practitioners and patients.
Research, however, is not simply an ivory tower or hospital-based pursuit useful for convincing the biomedical community and health care bureaucrats of what AOM practitioners "already know." Consider how "research" conjures up a sense of "looking again." In this context, research offers a framework within which to re-examine and reconsider how to improve clinical practice.
Experimental studies of electroacupuncture analgesia, for example, demonstrated that different frequencies of stimulation released different families of endogenous opioids (endorphins). These findings led to improved electrostim devices, now widely available for clinical practice, with a setting that automatically alternates between low and high frequency pulses, producing a more effective level of analgesia. Such studies that identify biochemical changes during acupoint stimulation have an overlooked value: they often awaken curiosity and confer legitimacy to acupuncture that does not occur in response to clinical trials alone. They describe acupuncture in a language with which the West is familiar. (Consider, in comparison, the difficulties with acceptance that homeopathy encounters, since despite recent rigorous clinical trials demonstrating its efficacy, there is no generally accepted scientific model to explain how it works.)
Research also provides a powerful approach for demonstrating that acupuncture should not be viewed (or tested) as a Western medical technique. The challenge is to design clinical trials that test acupuncture as it is delivered in clinical practice, with careful consideration of diagnostic subgroups and individualized treatment plans.
In this column, I will share my active commitment to research in ways that will help practicing acupuncturists become aware of research issues and research results. Research is a means for beginning conversations that will open doors to both wider acceptance and additional practice options. Consider, as examples, how such conversations can lead to increased opportunities in the areas of insurance reimbursement, referrals and hospital privileges. Future columns will provide updates and summaries of recently published AOM research as well as highlights from conferences where research relevant to this field is presented.