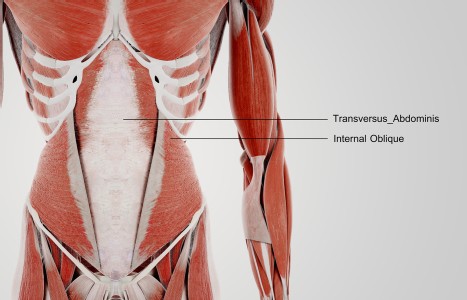
TrA-2, my primary needle location, I needle 95% of the time and I think it works the best. You’ll know you have the right point location when you discover the muscle twitching when applying electric stimulation.
Univ. of Virginia Receives $2.2 Million Grant to Study Echinacea
CHARLOTTESVILLE, VIRGINIA - First used by Native Americans centuries ago, echinacea has become one of the most popular herbal supplements on the market. According to the trade magazine Drug Store News, sales of echinacea reached approximately $72 million in 1999, making it the fifth best selling supplement in the United States that year.1 Part of echinacea's appeal is its reputed ability to fight the common cold, which strikes more than 60 million Americans each year, without causing unwanted side-effects. Other people use echinacea to prevent gingivitis, treat infections and cold sores, or boost the immune system.2
Exactly how echinacea works, and how effective it is in relieving cold symptoms, are significant points of contention that have yet to be resolved. Several variables can influence the effect echinacea has on the body, ranging from the part of the plant used to the conditions under which it is grown; the time of year it is harvested; and the manner in which it is processed. There are also three different species of echinacea, each with a different chemical composition.
Compounding matters even further is the fact that since echinacea is classified as a dietary supplement, it falls under the auspices of the Dietary Supplement Health Education Act, and is not subject to the same strict regulations or testing procedures the Food and Drug Administration requires of other products.
"If you buy product X off the shelf today and you go back six months later and buy the same brand, it may be completely different from the first thing you bought," explained Dr. Ronald Turner, a professor at the University of Virginia's department of pediatrics. "Echinacea and other supplements present a huge problem · because if you don't know what's actually in the product, studies on it can't be standardized, and therefore (you) cannot get consistent results."
That's why Turner and a group of colleagues from the University of Virginia Health System have received $2.2 million in funding from the National Institutes of Health.3 Their goal is to conduct a three-year clinical trial to establish whether echinacea really does help cure the common cold, and if so, what parts of the plant and manufacturing processes deliver the most effective product.
Turner's study will use one species of echinacea (e. angustofolia) that will be processed using three different extraction methods to produce different concentrations of various echinacea components. E. angustofolia was chosen because of its abundance in the U.S. Except for the extraction methods, the products will be identical. This process creates standardization among subject groups and ensures that all patients in the study receive the same type of product.
Approximately 450 volunteers will be recruited for the study and divided into two groups. Half of the volunteers will be given echinacea before being infected with the cold virus; the other half will receive echinacea after being infected. Some volunteers in both groups will receive a placebo so that their results can be compared with those receiving the actual supplement.
"One of the things we want to find out is whether echinacea has an effect on viral replication or on the body's inflammatory response," Turner said. "It may involve a combination of things."
The investigators expect to be finished with the trial part of the study by the end of 2003, and hope to have their results published in a major research journal after analyzing the data. For more information, contact the University of Virginia's Department of Pediatrics at (434) 924-5679.
References
- Eder R. Vitamin sales lose momentum, yet continue to increase. Drug Store News May 22, 2000, p. 66.
- Echinacea. HealthNotes Online (www.puritan.com/healthnotes1/Herb/Echinacea.htm).
- $2.2 million herbal cold remedy study underway at University of Virginia. Ascribe Newswire, August 27, 2002.