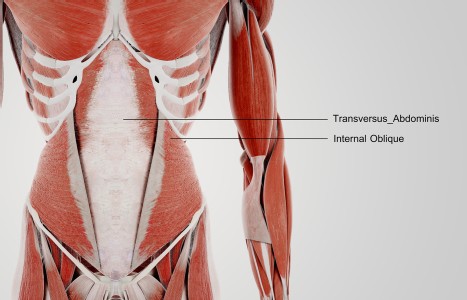
TrA-2, my primary needle location, I needle 95% of the time and I think it works the best. You’ll know you have the right point location when you discover the muscle twitching when applying electric stimulation.
When Big Pharma Meets Chinese Medicine
Earlier this year, Bayer made a media splash with their decision to buy the Dihon Pharmaceutical Group Co., a Chinese TCM manufacturer. While the move was primarily a strategic approach to achieve deeper penetration into the vast domestic healthcare market in China, it attracted the attention of Western practitioners of Chinese medicine, many of whom greeted the news with a mixture of interest and uncertainty.
Despite the fact that many practitioners are excited by the thought of increased funding and scientific validation of traditional therapies, a number of herbalists are concerned that the interest of large drug companies and the prospects of future prescription drugs from TCM products may affect the availability of such products for the non-MD community. Is there justification for such fears?
In recent years, a number of large Western companies have invested in Chinese medical research or products derived from TCM, including pharmaceutical giants such as Pfizer, Novartis, GSK, Merck, and Astrazeneca, as well as food and beverage conglomerates such as Coca-Cola and Nestle. At present, most of these companies have focused on using Chinese medicine to expand their market share in China, and relatively few companies have chosen to pursue FDA approval for botanical drugs. Although a few individual formulas such as dan shen di wan (known as "danshen dripping pill") and huang qin tang (known as "PHY906") are currently undergoing FDA trials for development as prescription drugs, thus far the only FDA-approved botanical drugs on the market remain derived from single plants rather than compound traditional formulas.
(At present, only two botanical drugs have passed the FDA approval process for sale in the USA. Veregen, a topical drug used to treat genital warts that is derived from sinecatechins found in green tea, became the first FDA approved botanical drug in 2006. Fulyzaq, a drug for the treatment of diarrhea in HIV patients, was approved in 2012; it is derived from the latex of a South American tree called Croton lechleri.)
In many ways, scientific interest in Chinese medical products is nothing new. Many famous drugs have been derived from Chinese herbal medicines, and new discoveries have continued to surface as technology has advanced. For example, in the late 1800s, ephedrine was successfully isolated from ma huang in Japan, which revolutionized the treatment of asthma in the early 20th century. Ephedrine, like many other botanically-derived alkaloid drugs, was isolated early on in the formative era of pharmacognosy. By the end of the 19th century, a number of other alkaloid drugs found in Chinese herbs had been successfully isolated, including aconitine, strychnine, brucine, atropine, scopolamine, and morphine.
More recently, artemisinin isolated from qing hao has proven to be a tremendous addition to the arsenal of drugs to treat malaria, one of the world's most challenging and lethal diseases. The discovery of artemisinin was facilitated by referencing traditional Chinese ben cao literature; inspired by Ge Hong's text Zhou Hou Bei Ji Fang (Emergency Formulas to Keep Up One's Sleeve), the Chinese researcher Tu Youyou noted that qing hao was used without the application of heat in the treatment of malaria, a key breakthrough. Today, Dr. Tu's achievement is honored with a Lasker Clinical Medicine Research Award, and artemisinin-based drugs are mainstream options in malaria treatment worldwide.
However, despite past success stories in the early isolation of botanical drugs and recently revised FDA regulations that facilitate botanical drug development, a number of hurdles continue to make it difficult to develop new plant-based drugs. In particular, one of the greatest challenges lies in ensuring batch-to-batch consistency, given that plant-based constituents tend to vary based on soil, weather conditions, harvest time, genetics, and many other complex factors. Additionally, identifying which constituents are responsible for specific pharmacological effects is very challenging, especially for formulas with multiple active ingredients or single herbs that have constituents that are technically difficult to analyze structurally, such as long-chain polysaccharides or compounds that lack commercially-available or affordable analytical reference standards. In such cases, developing quality control standards for new drugs is exceedingly difficult, and the ability to make dynamic customized formulas that are tailored to each individual patient will always remain a key strength of traditional medicine.
Although botanical drug development remains highly challenging, there is a huge financial incentive for pharmaceutical companies to continue pursuing botanical drugs that can be marketed to doctors and be reimbursed by insurance. On the positive side, each new success story will draw more public attention to traditional medicine, and will help sway skeptical doctors and consumers that require more scientific validation of traditional therapies. Fortunately, for traditional medical practitioners, natural products and ancient formulas like xiao yao san cannot be patented, so we are unlikely to lose access to our herbs. For example, the approval of Veregen in 2006 didn't affect access to green tea, nor did the arrival of artemisinin affect the availability of the crude herb qing hao.
A History of Assimilation of New Knowledge and Medicinal Materials
In past centuries, authors in Chinese medicine exhibited a tremendous degree of openness to new knowledge. For example, new theories based on human anatomy were pursued by famous historical doctors such as Wang Qing-Ren, the Qing Dynasty physician that created formulas such as xue fu zhu yu tang, and Li Shizhen recorded hundreds of new plants from local and foreign traditions in his monumental Ben Cao Gang Mu (Compendium of Materia Medica).
As China encountered new plants and new knowledge from local and foreign medicinals, some of the plants that were discovered have proven to be nearly impossible to duplicate with isolated pharmaceutical drugs, while others have been more successful. For example, the herb san qi (notoginseng) was originally a local medicinal that was first introduced into the Chinese medical literature by Li Shizhen in the 16th century. After its introduction, it gradually became an incredibly important medicinal, and, like other plants in the ginseng family, it has a complex chemical makeup that makes its effects nearly impossible to duplicate with isolated single constituents.
In other cases, the isolation and application of pure drugs has proven to be useful in medicine, particularly in the case of poisonous plants. For example, Li Shizhen recorded some of the first detailed descriptions of datura (man tuo luo) in China, which is a plant that is highly toxic and highly variable in its natural state. While Li noted its use in epilepsy and its applications in early anesthesia, datura was also used in China as a poison to induce a psychoactive delirium that would incapacitate a victim to facilitate robbery. Despite its profound medical effects, few herbalists would dare to use datura in its crude state today because only a thin line stands between dangerous toxicity and its therapeutic effects; by contrast, pure drugs such as atropine and scopolamine, when isolated from datura and administered at a precise, known dose, have made a great contribution to modern medicine.
In recent months, another previously local herb that recently entered Chinese medicine made the news in a big way- lei gong teng (Tripterygium wilfordii). Lei gong teng, which was used locally in China as a heat-clearing herb to target bi-syndromes, never appeared in classical formulas and has a relatively short history of traditional use.
However, it has profound pharmacological effects, and has received tremendous recent media attention for its ability to treat rheumatoid arthritis and pancreatic cancer. Unfortunately, it is also highly toxic to the liver, so researchers focused on new drug development have tried ingenious strategies to join its active ingredient with aptamers to form compound molecules that will help to allow it to specifically target cancer cells while avoiding healthy liver cells. This work, inspired by the traditional Chinese concept of formula construction, is something like a pharmaceutical equivalent of pairing a chief herb with a courier herb in a Chinese formula, where the courier helps direct the effects of the chief herb to the target site of the problem.
When it comes to toxic herbs like lei gong teng that lack a long history of established traditional use, there is value in new pharmaceutical approaches like the aptamer pairings, and there is a risk in assuming that natural products are inherently safe and superior. In our everyday experiences in clinic, practitioners of Chinese medicine frequently see cases where herbal therapy achieves effects that cannot be matched by drugs, especially in fields such as gynecology. Nonetheless, when it comes to highly toxic plants like lei gong teng, using precise doses and monitoring toxicity with lab results has merit, and the scientific approach complements the traditional approach.
Resources