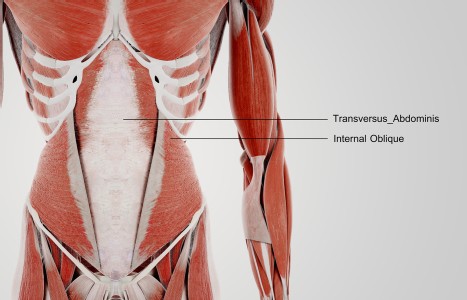
TrA-2, my primary needle location, I needle 95% of the time and I think it works the best. You’ll know you have the right point location when you discover the muscle twitching when applying electric stimulation.
Blue Poppy Herbs' Manufacturing Facility in China Certified by Independent Auditor as FDA GMP Compliant
March 25, 2009; Boulder, CO Blue Poppy Herbs' Chinese manufacturing facility in Hunan Province has successfully completed a GMP audit by NSF International, an independent, non-profit product and facility compliance auditing company with offices worldwide. At their website, NSF posts names and addresses of all manufacturing facilities that have successfully completed their GMP audit. NSF certifies that these companies are in compliance with Good Manufacturing Practices (GMP) as published by the FDA in May 2007.
"So far as we know, Blue Poppy is the first US provider of Chinese herbal medicine to require that its Chinese production facility meets these standards and be certified as such by an independent auditor," states Bruce Staff, General Manager at Blue Poppy. "The safety of our products and our customers is our first concern. We always want to be a source of positive product news coming from China."
Blue Poppy offers an extensive line of both standard and proprietary Chinese herbal formulas for sale through professional practitioner dispensaries. Product information can be obtained at [url=http://www.bluepoppy.com]http://www.bluepoppy.com[/url] www.bluepoppy.com. Interested parties may view the certificate of Hunan Nature Pharmaceutical at the NSF website, http://www.nsf.com/Certified/GMP/Listings.asp?CertName=Hunan.