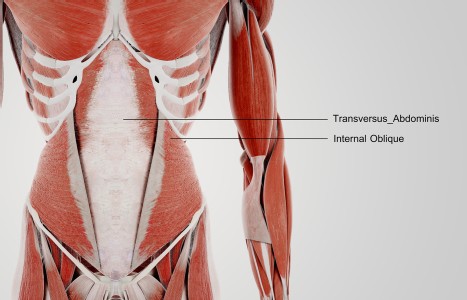
TrA-2, my primary needle location, I needle 95% of the time and I think it works the best. You’ll know you have the right point location when you discover the muscle twitching when applying electric stimulation.
Hatchet Jobs: Headlines, Studies and Big Pharma
We've been seeing the headlines from various sources: "Study: Supplements Fail to Ease Arthritis" (Seattle Post-Intelligencer), "Echinacea Has No Effect on Colds" (New York Times), "Study Debunks Echinacea's Powers" (CBS News) and "Supplements May Not Be Beneficial" (Marin Independent Journal). These headlines are referring to three recent studies involving glucosamine and chondroitin sulfate, saw palmetto and echinacea. These are just few examples of what seems like a major trend. Pick a superstar herb or supplement, perform a study, and hype the negative aspects in the media in order to discredit the herb/supplement (and by proximity, the whole alternative health movement). Let's take a closer look at what is happening, beginning with the studies and their subsequent media attention.
Echinacea was bashed last year in the form of a study published by The New England Journal of Medicine. In the American Botanical Council's response to the study, it was pointed out that the study had a major flaw in that the doses administered were extremely low. The therapeutic dose of E. angustifolia root, according to the WHO's monograph and the Canadian Natural Health Products Directorate, is 3,000 mg/day. The study used 900 mg/day. The study dose was 330 percent lower than the accepted recommended dose. Michael McGuffin, president of the American Herbal Products Association, on NPR's "All Things Considered," said, "It's like conducting a study on the effect of a third of an aspirin and then wondering why you still got a headache."
Now on to the chondroitin sulfate/glucosamine study. There were five groups: a placebo control; Celebrex; chondroitin alone; glucosamine alone; and chondroitin and glucosamine combined. There were no side effects in the chondroitin/glucosamine groups over the six-month study period. The control group showed 60 percent improvement; chondroitin/glucosamine, 67 percent improvement; and Celebrex, 70 percent improvement. However, when looking at those with moderate to severe symptoms, the picture changed; Celebrex had 69 percent effectiveness, while the combined group had 79 percent. This shows that for those with the worst symptoms, the supplements were better than Celebrex. Was this the headline? No. For arthritis sufferers, taking something that has no side effects and that isn't an NSAID (which puts over 100,000 people in the hospital every year and kills close to 20,000), Vioxx (a Cox-2 inhibitor that was pulled from the market) or Celebrex (which is also a Cox-2 inhibitor and is less effective for severe cases of arthritis), should be a cause to celebrate. Instead, the media took one aspect of the study, inflated and distorted it, and made sure to confuse the public, which doesn't read the entire article or look at the actual study. By the way, the researchers on this study were paid by Merck & Co. (the makers of Vioxx), Pfizer Inc. (manufacturers of Celebrex) and McNeil Consumer and Specialty Pharmaceuticals (who bring you Tylenol).
Saw palmetto was targeted in another study on benign prostatic hypertrophy (BPH). The men in this study were beyond stage 1 and stage 2 BPH (moderate to severe cases) and experienced severe adverse effects in the placebo group, suggesting these men had serious health issues beyond BPH. The preponderance of evidence for the effectiveness of saw palmetto is directed at men with mild symptoms. This is accepted by the WHO, the German Commission E, the Canadian government's Natural Health Products Directorate, and the monograph by the European Scientific Cooperative on Phytotherapy (ESCOP). Most Americans have never heard of these institutions, save perhaps the WHO, because our media does not interview or quote people from these organizations. Most Americans won't realize why this particular study does not negate the effectiveness of saw palmetto for mild to moderate symptoms of BPH.
Red yeast rice (aka hong qu, hong mi or chi qu) was targeted by Merck in the 1990s. Research on this substance by a Japanese researcher, Professor Endo, in the late 1970s led to the eventual isolation of what is now known as lovastatin. Essentially, it is the source of all of the statin drugs on the market today. In a landmark case of a private corporation trying to claim exclusive rights over a naturally occurring compound, a ruling was made after a back-and-forth legal battle that led the FDA to ban red yeast rice from the U.S. market, protecting Merck's patent. Because the yeast contained the compound known as lovastatin, the judge ruled that the natural product was an infringement against Merck. What is known is that the natural product contains multiple compounds that probably contribute in many ways to the cholesterol-lowering capabilities of the yeast. The natural compound is much more powerful at lowering cholesterol than the isolated lovastatin. A normal dose of yeast will yield approximately 7 mg of lovastatin, while an effective dose of Mevacor delivers 10 mg to 40 mg. Clearly, the natural compound has much more going for it than the isolated substance. Now the American public only has access to the expensive and potentially harmful statins, and is shut out from the age-old natural substance, which is affordable and much safer.
Now, about the FDA. On Dec. 15, 2003, at the Plaza Hotel in New York City, Daniel Troy, a lead council for the FDA under the Bush administration, offered to help drug companies torpedo certain lawsuits that claimed medications caused devastating and unexpected side effects. The FDA was batting for Big Pharma in a whole new way.
Troy is one of more than 100 high-level officials under Bush who once represented the very same industries they are now entrusted with the duty of regulating; in other words, lobbyists turned government watchdogs. Sen. Joe Lieberman, no liberal, likened the phenomenon to "the foxes guarding the foxes and the middle-class chickens getting plucked." The Department of the Interior's report on the FDA's ethics system called it "a train wreck waiting to happen."
It is no secret now that the highest officials in the FDA are in bed with the pharmaceutical companies. Even so, doctors are often without a clear and objective source of information on drug treatments because their only source of information comes from the drug companies themselves. A new study carried out by the Institute for Evidence-Based Medicine in Germany has found that 94 percent of the information contained in promotional literature sent to doctors by pharmaceutical companies has absolutely no basis in scientific fact. For example, as quoted in the study, medical guidelines from scientific groups are misquoted, the side effects of drugs are minimized, groups of patients are wrongly defined, study results are suppressed, treatment effects are exaggerated, risks are manipulated, and many effects of drugs are actually drawn from animal studies, not from human studies, even though the drugs are intended for human consumption.
Between the immensely powerful pharmaceutical interests, the pro-corporate Bush administration and the hype junkies in the media, herbs, natural products and supplements are getting the shaft. We have a culture battle here, pitting pure substances, drugs (which can be patented), versus natural substances (which cannot yet be patented, which contain a multitude of compounds that act synergistically, and which cannot be easily understood due to the complexity and variation of their makeup). Industry and science do not like variability in their subjects, and they can't make money off it. The culture war that subsumes this particular battle is too vast to address in this particular article, but suffice it to say that in many ways, this is about culture and not science.
I know that I'm preaching to the choir. What we as alternative health care providers need to do is be on the ball and be vigilant, so that whenever a big story breaks on the uselessness of a natural product, one that we know and love, we will get the real story out ASAP. Our patients will come in and ask us our opinion the very day the news breaks. The American Botanical Council is very responsive to these hatchet jobs and will be a great first source of rebuttal. Of course, one should read the actual study or the entire news story, not just the headline and the first few paragraphs. This will often clarify many anomalies for us. Write letters to the editor that very day, and back it up with facts. Big Pharma is not immune to bad publicity, and the public will respond to accurate information supporting what their experience and intuition tell them. The public needs to hear from us. Let's shout out the truth, loud and clear.