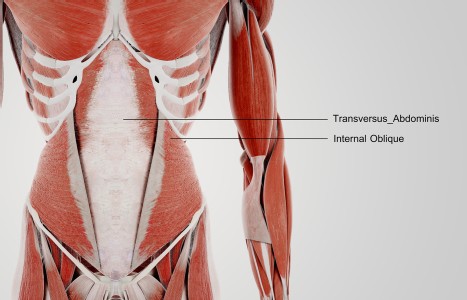
TrA-2, my primary needle location, I needle 95% of the time and I think it works the best. You’ll know you have the right point location when you discover the muscle twitching when applying electric stimulation.
NCCAM Chooses Electroacupuncture for First Intramural Study
The National Center for Complementary and Alternative Medicine (NCCAM) has begun recruiting patients for a phase II clinical trial to determine whether electroacupuncture reduces delayed nausea, which is often experienced by cancer patients following chemotherapy. The three-year trial is the first to be conducted by the center's Division of Intramural Research (DIR), which was established by NCCAM in April 2001.
Unlike chemotherapy-induced nausea, delayed nausea strikes between 1-5 days after completion of a chemotherapy session and can last several hours. The condition occurs most frequently in pediatric patients and is difficult to treat, leading to increased stress and possible weight loss. Cancer patients often are prescribed steroids to treat delayed nausea, but these drugs can cause weight gain and stunted growth, and leave patients more susceptible to infections.
Dr. Patrick Mansky, a research oncologist and staff clinician at NCCAM, will lead the investigation. "At present, we can treat the acute nausea that accompanies chemotherapy with conventional medications, but delayed nausea is tough to manage," acknowledged Dr. Mansky. "We hope that this trial will help reveal the value of electroacupuncture in managing delayed chemotherapy-induced nausea as well as other possible benefits."
Using acupuncture to treat nausea is not a new concept; as far back as 1997, the National Institutes of Health's Consensus Statement on Acupuncture listed acute nausea (which occurs within the first 24 hours after chemotherapy) as one of several conditions for which acupuncture could be useful. Delayed nausea has not been studied to the same degree, according to Dr. Marc Blackman, director of the DIR.
"The scientific evidence supporting use of electroacupuncture for relief of acute nausea following chemotherapy is very encouraging," noted Dr. Blackman. "Now we need to look at its potential utility for treating delayed post-chemotherapy nausea."
Dr. Stephen Straus, NCCAM's director, agrees with Dr. Blackman's assessment and thinks the study holds much promise for cancer patients. Finding an effective remedy for delayed nausea would not only enable these patients to take fewer drugs and avoid unwanted side-effects, but improve their quality of life.
"Our first intramural clinical trial addresses a significant problem for many cancer patients. If electroacupuncture does reduce delayed nausea following chemotherapy, oncologists will have a treatment option that may spare patients from negative side-effects associated with certain medications," said Dr. Straus.
In the study, a total of 52 patients between the ages of 16 and 35, who have been diagnosed with pediatric sarcoma and are starting their first-ever course of chemotherapy, will be randomized into two groups. The experimental group will receive electroacupuncture for seven consecutive days (twice daily on days one and two, once daily on days three through seven) starting with the first day of a chemotherapy cycle. This regimen will be repeated for a second cycle of chemotherapy. The researchers will then conduct followup tests on patients after two additional chemotherapy cycles. The control group will be given the same type of chemotherapy as the experimental patients, but will receive sham acupuncture.
Three licensed acupuncturists will coordinate and perform the needling procedures. Both investigators and patients, with the exception of the treating acupuncturist, will be blinded as to the type of care being delivered. The quantity and frequency of nausea episodes will be documented throughout the study, along with any side-effects that occur from treatment.
Two particular acupoints - P6, near the wrist, and St36, near the knee - will be used in the trial, based on their reported effectiveness in previous acupuncture studies. These points were chosen to not only determine the effectiveness of electroacupuncture, but to verify that P6 and St36 are effective in treating delayed nausea. As an additional control mechanism, both patient groups will also be needled at a common sham point.
Patient recruitment for the trial began at the NIH Clinical Center in Bethesda, Maryland, in May. The trial is expected to last three years, after which the data will be analyzed and compiled for possible publication. For additional information on the electroacupuncture trial or other complementary and alternative medicine trials being conducted by NCCAM, visit http://nccam.nih.gov/clinicaltrials or call (888) 644-6226.
Reference
- Electroacupuncture trial is NCCAM's first intramural study. FirstGOV for Seniors (www.seniors.gov/articles/0602/acupuncture.htm).