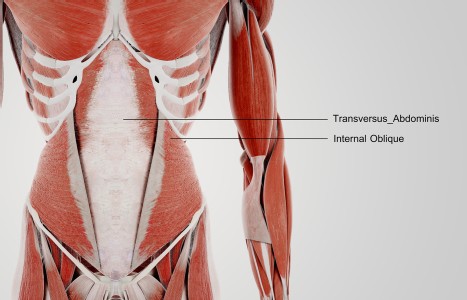
TrA-2, my primary needle location, I needle 95% of the time and I think it works the best. You’ll know you have the right point location when you discover the muscle twitching when applying electric stimulation.
Traditional Medicines Congress Extends Comment Period for Regulatory Model
The Traditional Medicines Congress has announced it has extended the deadline to receive comments regarding a draft proposal for the regulation of traditional medicines in the United States to June 30, 2006. The original deadline had been March 31, 2006, but the congress chose to extend it an additional three months after receiving several requests from herbalists and other health care providers to prolong the comment period, which would allow for a more comprehensive review of the document.
"The TM Congress has always intended to include any and all interested individuals and organizations in this essential review stage," commented Michael McGuffin, president of the American Herbal Products Association, one of the organizations that form the congress. "We encourage everyone with such an interest to read the draft and provide comments to let us know how it can be improved."

The Traditional Medicines Congress was formed in 2004 and is comprised of nine national organizations active in the field of traditional medicine, four of which are related directly to the practice of acupuncture and Oriental medicine. Its purpose is "to benefit public health by ensuring access to traditional medicines in a manner that provides a reasonable expectation of public safety."1
The draft proposal, "A Proposed Regulatory Model for Traditional Medicines: Guiding Assumptions and Key Components," was agreed upon by the members of the congress in October 2005, and made public the following month. According to the congress, the current definitions of "food" and "drug" contained in the Federal Food, Drug and Cosmetic Act are inadequate, as they do not provide a satisfactory regulatory category for traditional medicines. The proposed model calls for the creation of a "traditional medicine" category as a possible amendment to the act, which would accomplish several objectives, including the following:
- preserving access to traditional medicines that state and federal agencies have withdrawn from the market or may withdraw from the market;
- supporting the use of traditional medicines by health care providers and consumers;
- ensuring access to traditional medicines; and
- improving public safety by ensuring that accurate information about traditional medicine uses and indications is made public.
McGuffin added that the congress' draft proposal "would completely protect the current law while developing a new option that will benefit marketers who want to sell traditional medicines, and practitioners and consumers who want to use them."
In addition to extending the comment period, the congress has issued clarifications regarding three key aspects of its initial draft proposal:2
- The TM Congress is not suggesting that herbs now marketed as dietary supplements under the Dietary Supplement Health & Education Act be required to be sold as traditional medicines. Instead, the vision of the TM Congress is to create an optional new category. As envisioned, products in this new category could be labeled with their traditional uses (including medicinal uses), so long as such products conform to traditional criteria such as dose and preparation.
- The TM Congress is not attempting to influence the practice of medicine or the scope of practice of any therapeutic discipline. The TM Congress drafted its regulatory model to differentiate between products offered for retail sale and traditional medicines that are provided directly to a patient; for example, by an acupuncturist or an herbalist. The primary focus of the model is on the first of these, and attention has been given to the need to ensure that practitioners maintain direct control over traditional medicines that they produce for their patients.
- The TM Congress takes no position for or against license requirements for practitioners. Licensing for health care practitioners of every discipline is regulated on a state-by-state basis. The TM Congress has expressed neither support for, nor opposition to, expanding licensure requirements for any of the practitioners that use herbs in their practices, as this issue is outside of the scope of the Congress' purpose.
As of March 30, 2006, the congress has received more than 70 comments on the model from various traditional medicine providers. The current version of "A Proposed Regulatory Model for Traditional Medicines" is available online. General and/or specific comments regarding the document can be submitted to tmcongressfeedback@pobox.com.
References
- Traditional Medicines Congress calls for comments on "ideal regulatory model." Acupuncture Today, January 2006.
- TM Congress extends comment period for draft model. NutraIngdients.com press release, March 30, 2006.